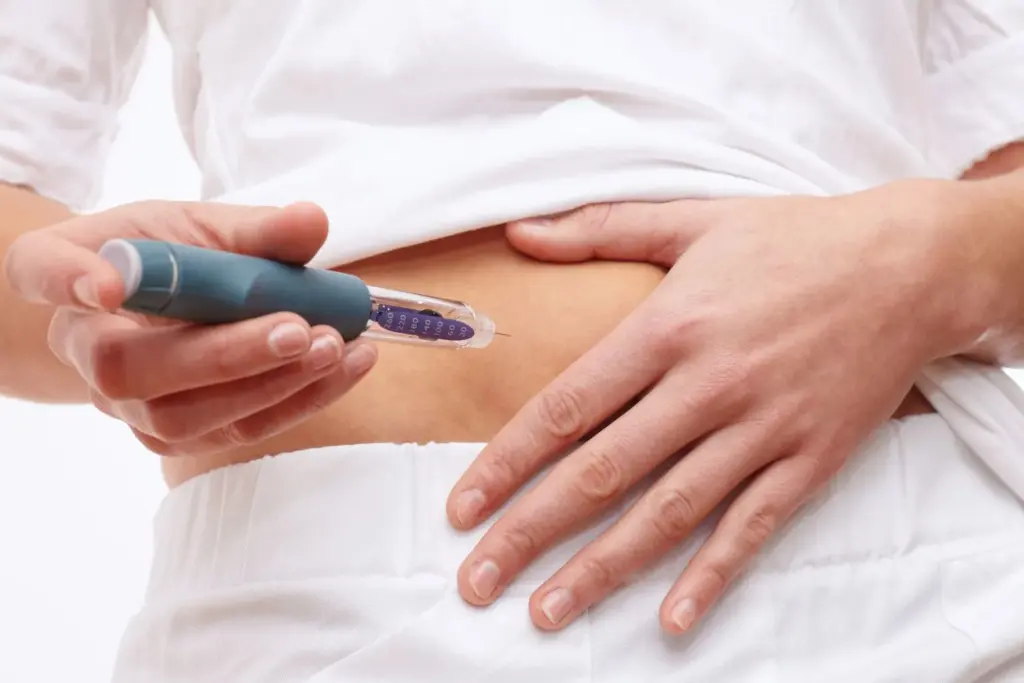
Ozempic in India: Indian drugmaker Dr Reddy’s Laboratories said it has received approval from the country’s drug regulator to manufacture and sell a generic version of Ozempic, the diabetes injection whose active ingredient is semaglutide.
The company said it is planning to sell about 12 million semaglutide injectable pens in the first year after launch, targeting a market that has been expanding rapidly as demand grows for newer diabetes therapies that are also used off-label for weight loss.
Also Read | A guide to GLP-1 side effects: What to expect and how to manage them
Semaglutide is the active ingredient in both Ozempic and Wegovy. The patent is due to expire in March 2026, opening the door for generic launches in India and other markets. Dr Reddy’s said it has approval for the diabetes indication but is still awaiting a separate clearance for the obesity drug Wegovy.
Executives said the company plans to work with local partners for the India rollout and that it has enough manufacturing capacity to meet expected demand. Dr Reddy’s also said it is preparing to introduce semaglutide in Canada this year and expand into other emerging markets.
The update came as the company reported quarterly earnings showing a smaller-than-expected decline in profit. Consolidated net profit fell 14.4% to 12.1 billion rupees for the quarter ended December 31, while revenue from operations rose 4.4% to 87.53 billion rupees.
Revenue from Dr Reddy’s India business climbed 19% to 16.03 billion rupees, supported by price increases and contributions from the anti-vertigo brand Stugeron, which the company acquired in September.
The profit drop, the first in five quarters, was largely attributed to the weaker performance of lenalidomide, the generic version of Revlimid, amid pricing pressure and competition in the United States.